

Q1 2021 Financial Results May 5, 2021

Forward Looking Statements This presentation contains forward-looking statements that involve risks and uncertainties. Such forward-looking statements reflect Baudax Bio’s expectations about its future performance and opportunities that involve substantial risks and uncertainties. When used herein, the words “anticipate,” “believe,” “estimate,” “may,” “upcoming,” “plan,” “target,” “goal,” “intend,” and “expect,” and similar expressions, as they relate to Baudax Bio or its management, are intended to identify such forward-looking statements. These forward-looking statements are based on information available to Baudax Bio as of the date of publication on this internet site and are subject to a number of risks, uncertainties, and other factors that could cause Baudax Bio’s performance to differ materially from those expressed in, or implied by, these forward-looking statements. These forward-looking statements are subject to risks and uncertainties including, among other things, the completion of the registered direct offering and the intended use of proceeds from the registered direct offering, the ongoing economic and social consequences of the COVID-19 pandemic, including any adverse impact on the commercial launch of ANJESO® or disruption in supply chain, Baudax Bio’s ability to maintain regulatory approval for ANJESO, Baudax Bio’s ability to successfully commercialize ANJESO; the acceptance of ANJESO by the medical community, including physicians, patients, health care providers and hospital formularies; Baudax Bio’s ability and that of Baudax Bio’s third party manufacturers to successfully scale-up our commercial manufacturing process for ANJESO, Baudax Bio’s ability to produce commercial supply in quantities and quality sufficient to satisfy market demand for ANJESO, Baudax Bio’s ability to raise future financing for continued product development, payment of milestones and ANJESO commercialization, Baudax Bio’s ability to pay its debt and satisfy conditions necessary to access future tranches of debt, Baudax Bio’s ability to comply with the financial and other covenants under its credit facility, Baudax Bio’s ability to manage costs and execute on our operational and budget plans, the accuracy of Baudax Bio’s estimates of the potential market for ANJESO, Baudax Bio’s ability to achieve its financial goals; and Baudax Bio’s ability to obtain, maintain and successfully enforce adequate patent and other intellectual property protection. These forward-looking statements should be considered together with the risks and uncertainties that may affect Baudax Bio’s business and future results included in Baudax Bio’s filings with the Securities and Exchange Commission at www.sec.gov. These forward-looking statements are based on information currently available to Baudax Bio, and Baudax Bio assumes no obligation to update any forward-looking statements except as required by applicable law.
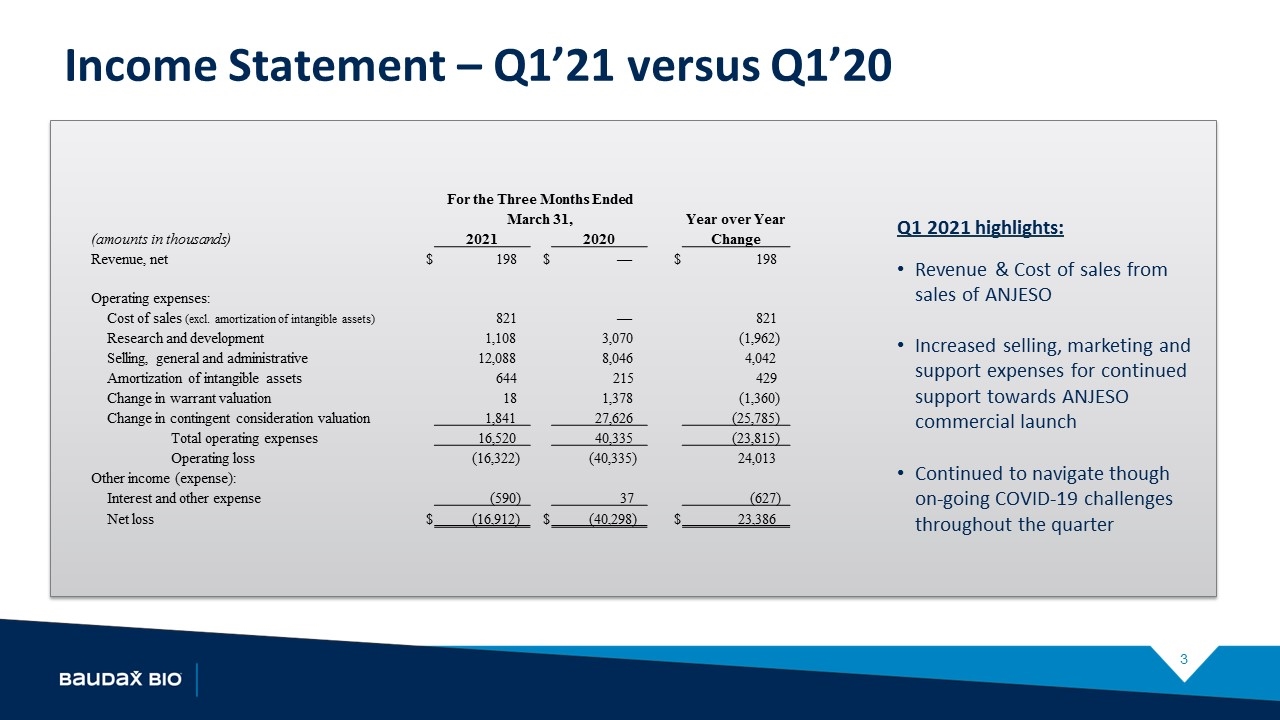
Income Statement – Q1’21 versus Q1’20 Q1 2021 highlights: Revenue & Cost of sales from sales of ANJESO Increased selling, marketing and support expenses for continued support towards ANJESO commercial launch Continued to navigate though on-going COVID-19 challenges throughout the quarter For the Three Months Ended March 31, Year over Year (amounts in thousands) 2021 2020 Change Revenue, net $ 198 $ — $ 198 Operating expenses: Cost of sales (excl. amortization of intangible assets) 821 — 821 Research and development 1,108 3,070 (1,962) Selling, general and administrative 12,088 8,046 4,042 Amortization of intangible assets 644 215 429 Change in warrant valuation 18 1,378 (1,360) Change in contingent consideration valuation 1,841 27,626 (25,785) Total operating expenses 16,520 40,335 (23,815) Operating loss (16,322) (40,335) 24,013 Other income (expense): Interest and other expense (590) 37 (627) Net loss $ (16,912) $ (40,298) $ 23,386

Baudax Bio: Q1’21 Anjeso Status Update Launch Progress is being made despite varying levels of COVID-19 disruption of account access, formulary meetings, and elective surgeries Vials sold grew ~40% in Q1’21 compared to Q4’20 Sales to existing hospitals and ASC accounts doubled from Q4’20 to Q1’21 End user reorders at a 59% rate in Q1’21
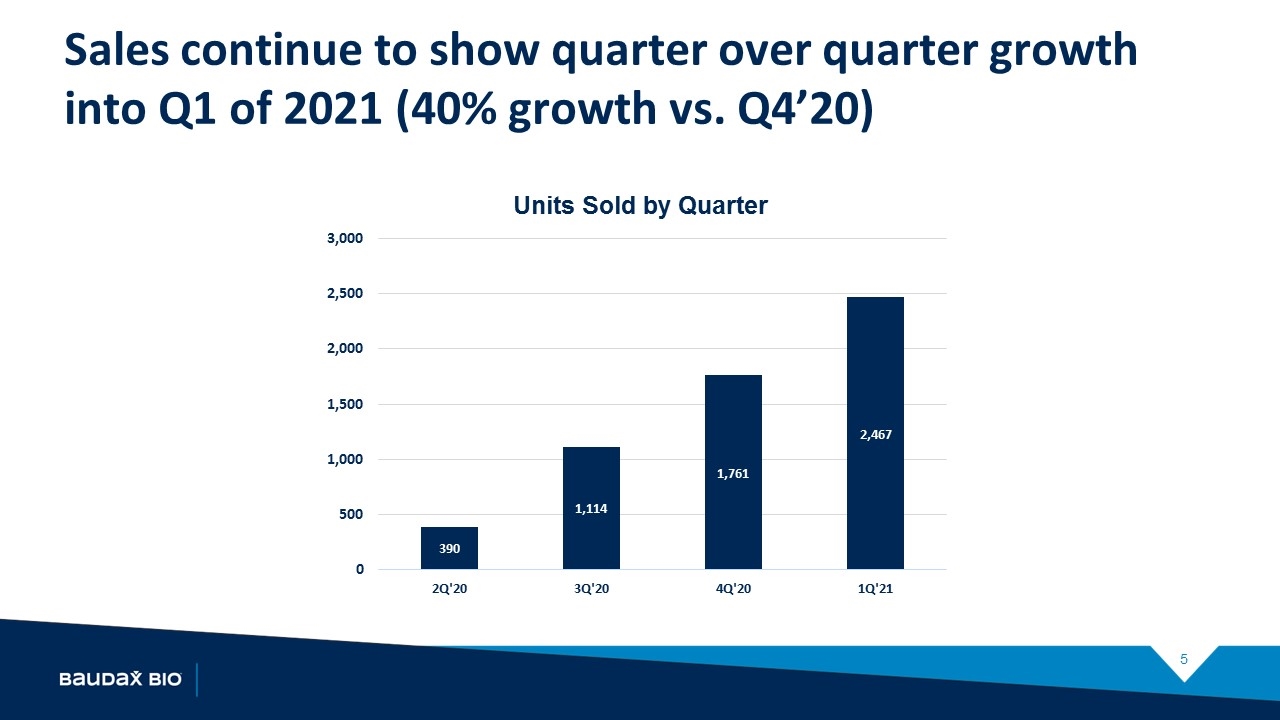
Sales continue to show quarter over quarter growth into Q1 of 2021 (40% growth vs. Q4’20)
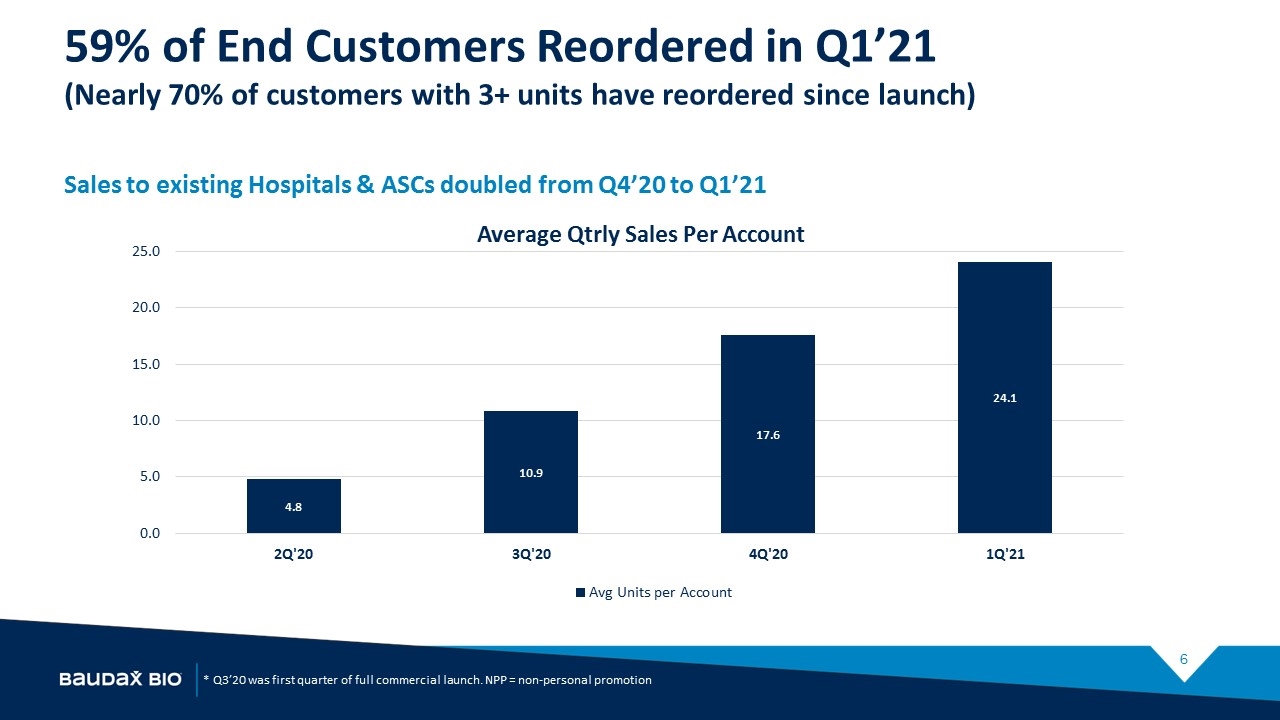
59% of End Customers Reordered in Q1’21 (Nearly 70% of customers with 3+ units have reordered since launch) Sales to existing Hospitals & ASCs doubled from Q4’20 to Q1’21 * Q3’20 was first quarter of full commercial launch. NPP = non-personal promotion
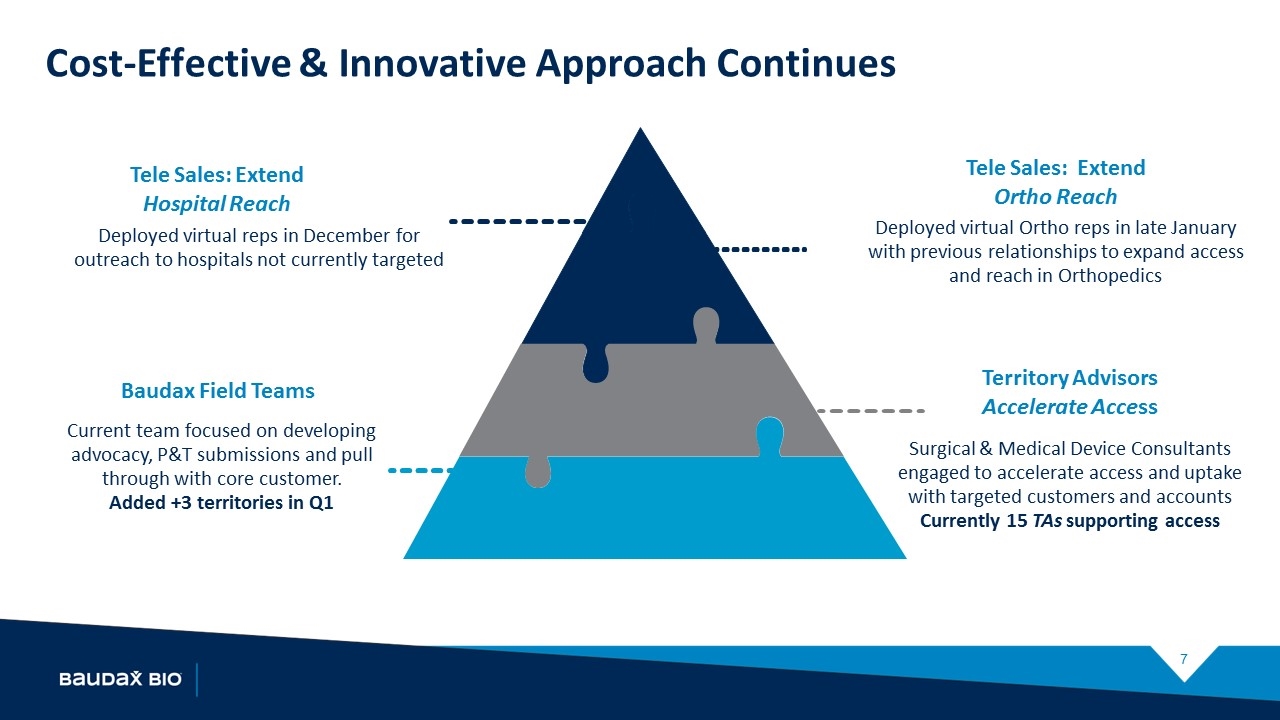
Current team focused on developing advocacy, P&T submissions and pull through with core customer. Added +3 territories in Q1 Baudax Field Teams Deployed virtual reps in December for outreach to hospitals not currently targeted Tele Sales: Extend Hospital Reach Surgical & Medical Device Consultants engaged to accelerate access and uptake with targeted customers and accounts Currently 15 TAs supporting access Territory Advisors Accelerate Access Deployed virtual Ortho reps in late January with previous relationships to expand access and reach in Orthopedics Tele Sales: Extend Ortho Reach Cost-Effective & Innovative Approach Continues

Commercial Launch Highlights Q1’21 Increasing Awareness In A Cost-Efficient Way Vial Use Growing Quarterly ANJESO ‘On Formulary’ at approximately 90 accounts at the end of Q1 Largest areas of usage remains hard tissue procedures including orthopedics, and podiatry; plastic surgery and soft tissue procedures gaining momentum COVID Continue to Impact Rate of Update COVID-19 continues to impact customer access and delays in formulary reviews Delays in order set implementation has also impacted faster growth in ‘On Formulary’ accounts NPP = non-personal promotion Field focused remains on formulary reviews, pull-through & expanding usage within accounts Team supplemented with tele-sales team, territory advisors & hyper-targeted NPP Internal teams expanded to support areas where momentum has developed